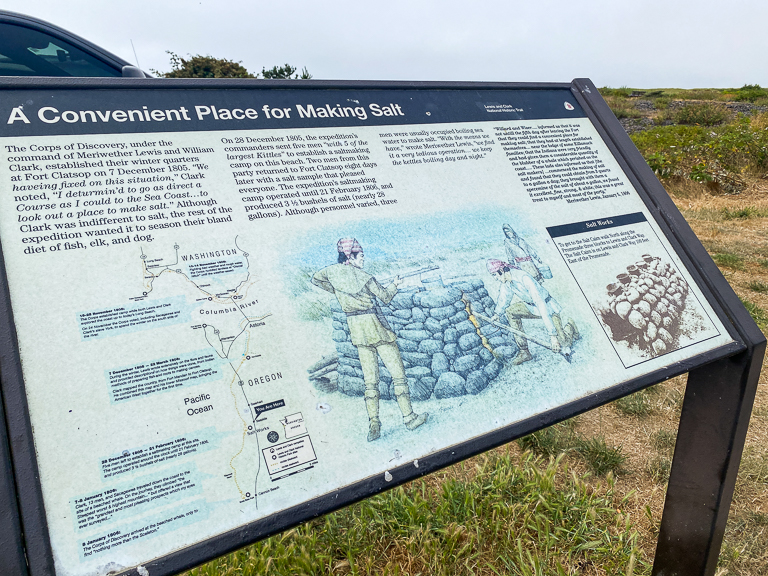
Welcome to Seaside Oregon! While walking along the Strand by the beach, we noticed that just off the path was a very very small National Park Historic Monument. It's the Lewis and Clark Salt Works!
Alli - Daddy, can we make salt?
You know what? That sounds like a fun, hands-on learning experience. Bringing history alive. Let's do it!
We gathered as many containers as we could find, rinsed them out well, and headed to the ocean to fill them up. All told, we had about 6 gallons I'd estimate.
I didn't have a large boiling vessel, so we hit Goodwill to find something appropriate. And I didn't want to risk messing up one of our nice pots. The electric burner is going to come in useful to keep me from having to burn propane to power this thing.
The seawater got filtered through paper towels before boiling to filter out and large particulates. The dissolved salts should pass right through.
There's actually a good reason the Lewis & Clark expedition went so far to the south from Fort Clatsop to establish the salt works. Their party was following the path of the Columbia River, a fresh water river, and when it met up with the ocean the two waters mix and the amount of salt in the water is diluted.
Full pot, which will need to be refilled a few times.
And now we wait.
Because this was outside, I made sure to cover it loosely to prevent anything from falling into the top. There's nearby trees, and as we were getting set up, we could tell something would need to be done. Otherwise this might have a boiled evergreen flavor!
I'll save you all the in-between steps. This was a very long process. We don't have rip roaring fires like the Salt Works had. We just have a small stovetop burner that takes a while to transfer the heat to the water. As the water got lower in the pot, I would add more, and it would start the boiling process all over again. And looking into the water each time I added it, I still wasn't seeing salt crystals. That is until day 4 when I was all out of water to add and let it start boiling all the water away.
There we go! That's the salt we've been looking for!
Ian and Alli were excited to see the results of their homemade salt experiment!
It is possible to burn salt, according to what I read, so I turned the temperature to low to hopefully get some of this excess water out.
I then transferred it to a parchment lined cookie sheet.
And to speed up the dehydration process, put it in our oven at 125F until things started to dry out.
And success! We have salt crystals!
Congratulations kids! You just made your own salt! Just like the Lewis and Clark Expedition!
Giving it a taste. Yep! That's salty alright! Yum!
Our 6 gallons of water boiled down to make almost a pound and a half, 21 ounces, of salt.





















Bringing history to life!...great suggestion by Alli to make salt from the sea water. That was quite a resourceful salt-making setup...best of all, it worked and the kids had fun learning about the process. Memories of this salt-making experiment will be a great reminder of what they saw on part of their Oregon visit. As an aside, hope Alli's "banged up" right knee is okay now...the skin discoloration and bandage hinted at some mishap. EOM
ReplyDeleteWe were just discussing Lewis and Clark and their making salt in Seaside. We are kind of Lewis and Clark nerds having followed the trail from Long Beach, WA to Three Forks, MT and we decided salt making would be a great family project. My “kids” are 37 and 41 but we still like family “adventures”. Thanks for sharing your story and insights!
ReplyDeleteThis is such a great example of bringing history, and the actually ability to take of one's self, to life. Inspiring!!
ReplyDeleteSince you filtered the water through paper towels AND boiled the saltwater to remove the water, the salt should be reasonably safe to use. I'm no moledular biologist, but that's my thought. Thanx for sharing!
ReplyDelete